| Translate This News In |
|---|
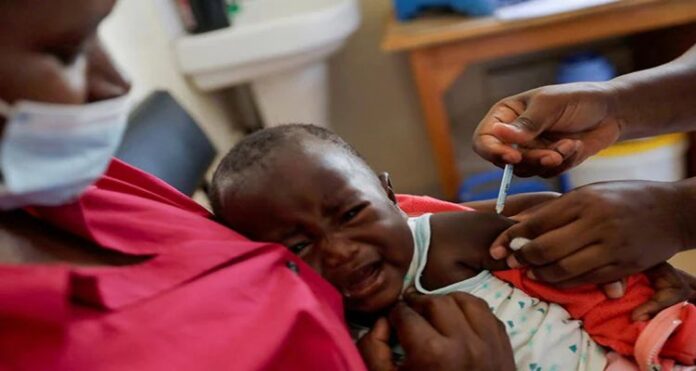
Last year, the World Health Organization endorsed the first-ever malaria vaccine, a historic milestone that promised to bring an end to a disease that kills a child every minute. In reality, efforts are falling far short of that, with GSK Plc’s capacity to produce as many doses of its shot as needed to be hampered by a lack of funding and commercial potential, according to Reuter’s interview sessions with about a dozen WHO officials, GSK staff, scientists, and non-profit groups.
Following 2019 pilot programs, the British drugmaker committed to producing up to 15 million doses per year through 2028, which is significantly less than what the WHO estimates is required. According to a person familiar to the company, it is currently unlikely to earn more than a few million dollars per year before 2026.
A GSK spokesperson told Reuters that the company could not produce enough Mosquirix vaccine to meet the high demand without additional funding from international donors, but did not specify how many doses it anticipated to deliver annually in the first years of the roll-out.
“Demand will probably outstrip current supply forecasts over the next five to ten years,” said Thomas Breuer, GSK’s chief global health officer.
In a large-scale clinical trial, the vaccine’s effectiveness in preventing severe cases of malaria in children was estimated to be around 30%. Some officials and campaign contributors are hoping that a second shot at getting tested by Oxford University will be better, less expensive, and easier to produce in large quantities.
Many Africans are disappointed by the world’s inability to fund more Mosquirix shots. Children on the landmasses account for the vast majority of the 600,000 malaria deaths worldwide each year.
“Mosquirix has the possibilities to save a lot of precious children before another new vaccine comes along,” said Kwame Amponsa-Achiano, a public health consultant in charge of a pilot vaccination programme in Ghana. “The longer we wait, the more children will perish needlessly.”
Rebecca Adhiambo Kwanya of Kisumu, Kenya, needs no persuading: her four-year-old child Betrun has had numerous malaria bouts since birth, but her 18-month-old Bradley, who was vaccinated in the pilot program, has not.
The lack of international appetite for producing and distributing more Mosquirix contrasts sharply with the record speed and resources with which wealthy countries managed to secure vaccines for COVID-19, a disease that poses little risk to children.
Unlike many pharmaceuticals, there is no significant market for a malaria vaccine in industrialized nations, where drug companies typically make large profits that they claim allow them to sell their products at much lower prices in poorer countries.